Introduction
Pre-filled inhalers packaging plays a critical role in the delivery of respiratory medications, offering convenience, dosage accuracy, and improved patient compliance. These packaging solutions are designed to house metered-dose inhalers (MDIs), dry powder inhalers (DPIs), nebulizers, and nasal sprays, ensuring product integrity and ease of use. As respiratory diseases such as asthma and COPD continue to rise globally, the demand for efficient, safe, and user-friendly inhaler packaging is accelerating.
The Evolution
The evolution of inhaler packaging began with basic canisters and manual inhalers. Over time, the need for precise dosing, portability, and hygiene led to the development of pre-filled, tamper-evident, and child-resistant packaging formats. Innovations in materials science introduced lightweight polymers, barrier films, and moisture-resistant coatings.
Today’s packaging integrates smart features such as dose counters, ergonomic designs, and compatibility with digital health platforms. Manufacturers are also focusing on sustainability by adopting recyclable materials and reducing carbon footprints in production.
Market Trends
Rising Prevalence of Respiratory Disorders: Increasing cases of asthma, COPD, and allergic rhinitis are driving demand for pre-filled inhalers and their packaging.
Shift Toward Patient-Centric Design: Packaging is being optimized for ease of use, especially for pediatric and geriatric populations.
Growth in Biologics and Combination Therapies: Complex formulations require advanced packaging to maintain stability and efficacy.
Sustainable Packaging Initiatives: Eco-friendly materials and reduced plastic usage are gaining traction among manufacturers and regulators.
Expansion of Home Healthcare: The rise in self-administered therapies boosts demand for intuitive, pre-filled inhaler packaging.
Challenges
High Manufacturing Costs: Advanced packaging materials and precision filling technologies increase production expenses.
Regulatory Compliance: Stringent standards for drug-device combination products require rigorous testing and documentation.
Material Compatibility Issues: Ensuring chemical stability between drug formulations and packaging materials remains a technical hurdle.
Supply Chain Disruptions: Global logistics challenges and raw material shortages can delay production and distribution.
Risk of Contamination or Misuse: Improper sealing or labeling can compromise patient safety and product efficacy.
Market Scope
The pre-filled inhalers packaging market includes:
By Product:
Nasal Inhalers
Metered Dose Inhalers (MDIs)
Dry Powder Inhalers (DPIs)
Nebulizers
By Raw Material:
Plastics & Polymers (HDPE, LDPE, PET, PVC)
Paper & Paperboards
Glass
Metals
By Dose Type:
Single Dose
Multiple Dose
By Application:
Wet or Liquid Drug
Dry Powder Drug
End users include pharmaceutical companies, hospitals, clinics, and home care providers. Packaging formats range from primary containers (canisters, cartridges) to secondary packaging (cartons, labels) and tertiary solutions for bulk transport.
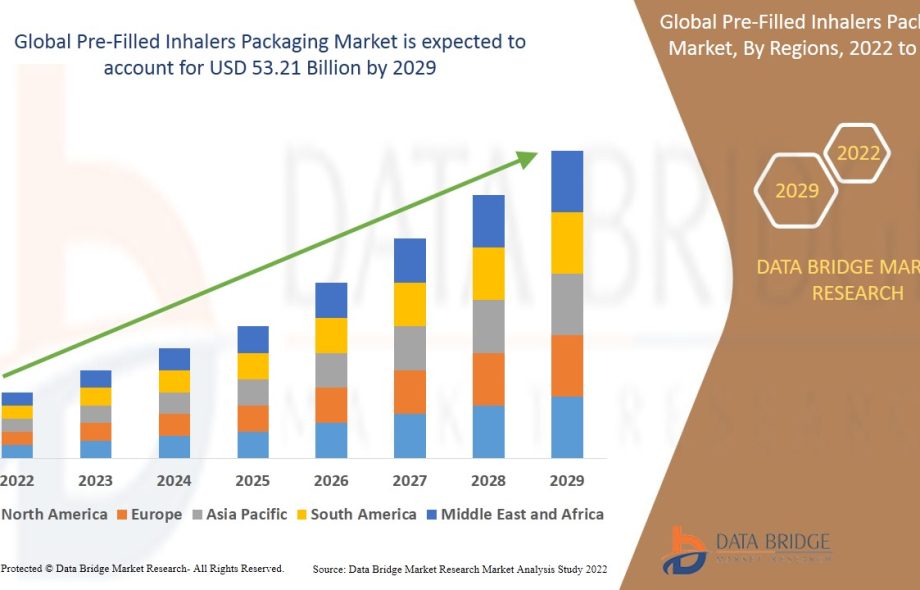
Market Size
According to industry analysis, the global pre-filled inhalers packaging market was valued at approximately USD 53.21 billion in 2024, with a projected CAGR of 8.1% through 2029. Growth is driven by rising respiratory disease incidence, expanding pharmaceutical production, and technological advancements in packaging.
Asia-Pacific leads in growth rate due to increasing healthcare access, urbanization, and government initiatives. North America and Europe maintain strong market shares due to established pharmaceutical infrastructure and regulatory frameworks.
Key Players
GlaxoSmithKline plc
Cipla Inc.
3M
Beximco Pharmaceuticals Ltd
H&T Presspart Manufacturing Ltd.
Summit Packaging Solutions
Transcendia
AUER Packaging
International Plastics Inc.
Kiva Container
Factors Driving Growth
Increased Respiratory Disease Burden: Urban pollution, smoking, and aging populations contribute to rising inhaler use.
Technological Advancements: Smart inhalers and connected packaging enhance adherence and monitoring.
Pharmaceutical Industry Expansion: Growth in generics and biologics fuels demand for differentiated packaging.
Consumer Preference for Convenience: Pre-filled, ready-to-use formats improve patient experience and reduce dosing errors.
Regulatory Push for Safety and Sustainability: Compliance with global standards encourages innovation in materials and design.
Other Trending Reports
https://www.databridgemarketresearch.com/reports/global-veterinary-artificial-insemination-market
https://www.databridgemarketresearch.com/reports/global-wall-bed-market
https://www.databridgemarketresearch.com/reports/global-wakeboarding-equipment-market
https://www.databridgemarketresearch.com/reports/global-virtual-desktop-infrastructure-vdi-market
https://www.databridgemarketresearch.com/reports/global-vegetable-oil-market
https://www.databridgemarketresearch.com/reports/global-urology-medical-device-market
https://www.databridgemarketresearch.com/reports/global-synthetic-compressor-oil-market
https://www.databridgemarketresearch.com/reports/global-sustainable-air-filters-market
https://www.databridgemarketresearch.com/reports/global-surgical-glue-market
https://www.databridgemarketresearch.com/reports/global-strapping-equipment-market
 :
https://in.pinterest.com/vikaskokate111201/
:
https://in.pinterest.com/vikaskokate111201/